
Home | Group | Research | Publications | Seminars/Workshops | Teaching | Open Positions | How to find us | Intranet
Deutsch | English

Home | Group | Research | Publications | Seminars/Workshops | Teaching | Open Positions | How to find us | Intranet
Deutsch | English

The selective cleavage or well-defined formation of bonds is the key issue of chemical synthesis. Such processes may be initiated by controlled changes of the molecular electron density distribution. Electron-molecule interactions offer an approach to control the electronic structure and thus the reactivity of molecules. They prepare molecules in different states depending on the initial kinetic energy of the electron. In consequence, a wide range of different chemistries can be initiated by electron-molecule interactions.
The changes in electronic structure are particularly large when a charge is added to the molecule or removed from it. Already electrons with near-thermal energy can be trapped by a molecule yielding reactive short-lived radical anions that dissociate different bonds depending on the energy of the electron. The fragments resulting from such dissociative electron attachment (DEA) initiate subsequent reactions that lead to the formation of new products.
Above the ionization threshold an electron can be removed from a molecule. The resulting radical cation can also dissociate. However, if it remains intact, it can add as such to electron rich reaction partners. Such reactions are favored by the Coulomb force between the radical cation and the nucleophil. Recombination with a thermalized electron yields the neutral product.
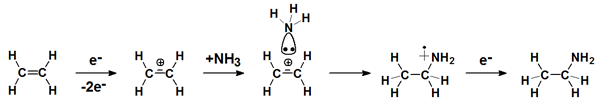
This type of reaction is highly appealing because the intact radical cation is the largest possible reactive species and thus can induce syntheses that may be classified as atom-efficient. These syntheses are relevant to areas as diverse as surface functionalisation and astrochemistry.
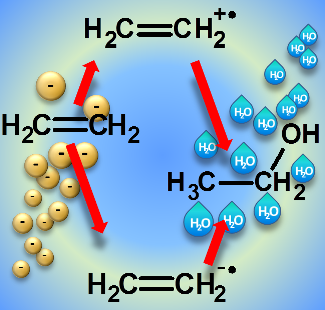
In specific cases, electron attachment can also trigger reactions that lead to the formation of the same product as obtained through an ionization-driven mechanism. Ethanol is thus formed in mixtures of water and ethylene both through an ionization-driven reaction and as consequence of electron attachment to ethylene.
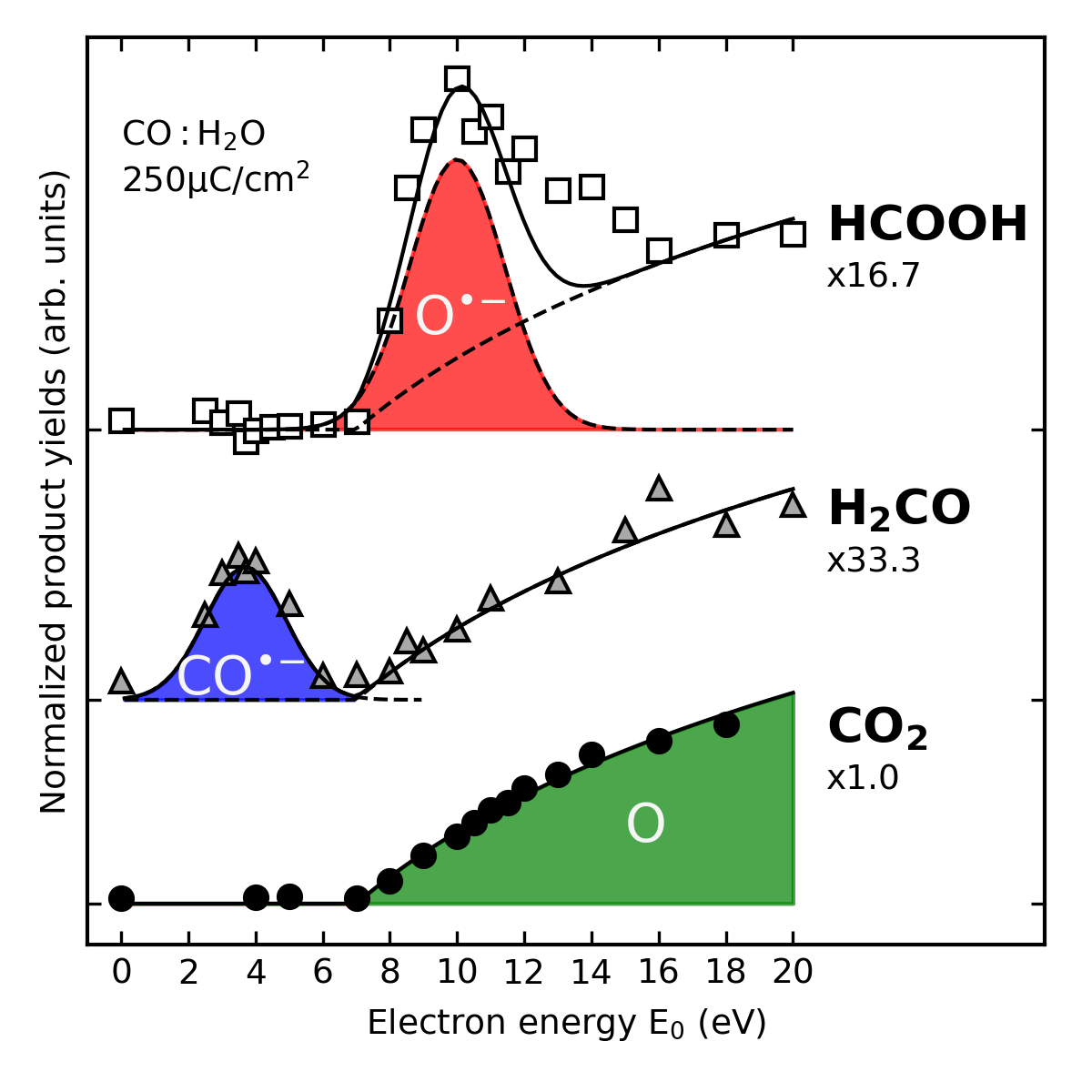
The energy of the impinging electrons determines the reactive fragments that are formed and thus also the final products. Experiments on ice mixtures of CO and H2O performed with sufficiently small steps on the energy scale reveal that formaldehyde and formic acid are produced within well-defined energy ranges. Different anionic intermediates are involved in these reactions. In contrast, the lack of resonant behavior and the low threshold for formation support that CO2 results from reaction of CO with neutral O.
More information:
(1) Mechanisms of methyl formate production during electron-induced processing of methanol–carbon monoxide ices (2021 PCCP HOT article);
F. Schmidt, P. Swiderek, J. H. Bredehöft,
Phys. Chem. Chem. Phys. 23, 11649-11662 (2021).
(2) Electron-Induced Processing of Methanol Ice;
F. Schmidt, P. Swiderek, J. H. Bredehöft,
ASC Earth Space Chem. 5, 391–408 (2021).
(3) Formation of Formic Acid, Formaldehyde, and Carbon Dioxide by Electron-Induced Chemistry in Ices of Water and Carbon Monoxide; F. Schmidt, P. Swiderek, J. H. Bredehöft, ASC Earth Space Chem. 3, 1974 (2019).
(4) Electron-induced formation of ethyl methyl ether in condensed mixtures of methanol and ethylene; F. Schmidt, P. Swiderek, J.H. Bredehöft, J. Phys. Chem. A 123, 37 (2019).
(5) Electron-induced synthesis of formamide in condensed mixtures of carbon monoxide and ammonia;
J.H.Bredehöft, E.Böhler, F. Schmidt T. Borrmann, P. Swiderek, ACS Earth Space Chem. 1, 50 (2017).
(6) Electron-induced hydration of an alkene: Alternative reaction pathways;
J. Warneke, Z. Wang, P. Swiderek, J.H. Bredehöft, Angew. Chem. Int. Ed. 54, 4397 (2015).
(7) Low-energy electron-induced hydroamination reactions between different amines and olefins;
E. Böhler, J.H. Bredehöft, P. Swiderek, J. Phys. Chem. C 118, 6922 (2014).
(8) Control of chemical reactions and synthesis by low-energy electrons;
E. Böhler, J. Warneke, P. Swiderek, Chem. Soc. Rev. 42, 9219 (2013).
(9) Reactions and anion desorption induced by low-energy electron exposure of condensed acetonitrile;
A.D. Bass, J.H. Bredehöft, E. Böhler, L. Sanche, P. Swiderek, Eur. Phys. J. D 66, 53 (2012).
(10) Elektronenstrahlen – neue Wege zur Reaktionskontrolle;
P. Swiderek; Nachrichten aus der Chemie 60, 1078 (2012).
(11) Low-energy electron-induced hydroamination of an alkene;
T.Hamann, E.Böhler, P.Swiderek, Angew. Chem. Int. Ed. 48, 4643 (2009).
The images from top to bottom are taken from articles (8), (6) und (3).